Get the App
For Doctors
Login/Sign-up
measles-vaccine-live Health Feed
Asked for male, 32 years old from Gorakhpur
Share
Bookmark
Report
Asked for female, 36 years old from Bangalore
Share
Bookmark
Report
Asked for male, 39 years old from Allahabad
Share
Bookmark
Report
Asked for female, 24 years old from Kannur
Share
Bookmark
Report
If germs have already entered (means u r already infected), then no vaccination will not help. But if u r still germs free, then yes vaccination will work
Last Updated: 6 years ago• Featured Tip
Share
Bookmark
Report
Chickenpox or varicella is a type of viral infection that causes itchy rashes accompanied by tiny fluid-filled blisters. It is highly communicable to those who have not experienced this disease earlier or have not been immunized against it through vaccination.
The vaccine against chicken pox is a shot protecting anyone, who has already contracted the disease. It is known as the varicella vaccine since chicken pox is triggered by the virus called varicella-zoster. The vaccine is prepare...more
The vaccine against chicken pox is a shot protecting anyone, who has already contracted the disease. It is known as the varicella vaccine since chicken pox is triggered by the virus called varicella-zoster. The vaccine is prepare...more
Last Updated: 7 years ago• Featured Tip
Share
Bookmark
Report
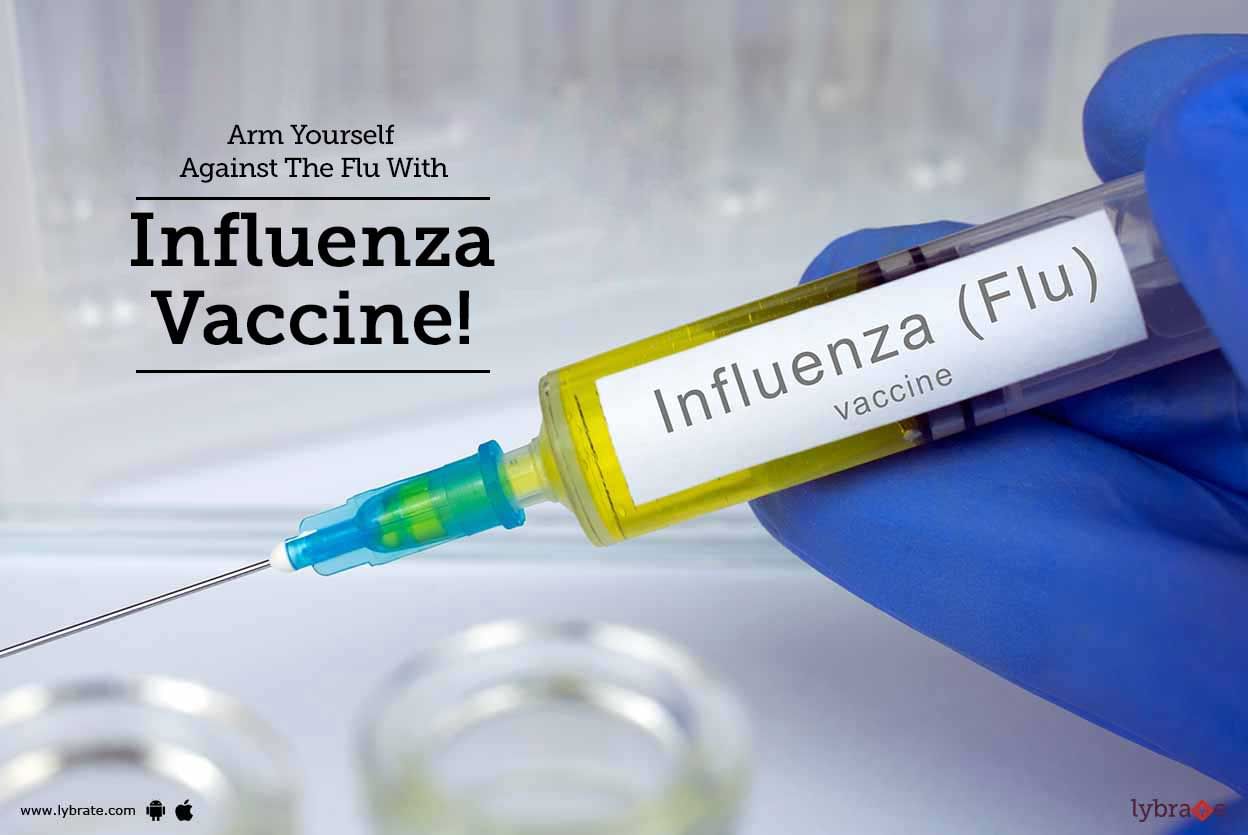
Flu is caused by a virus known as influenza and is a common respiratory disease. It is typically caused from the month of October to May. Some of the common symptoms include fatigue, runny nose, fever, cough, muscle ache, sore throat, body ache, etc.
The flu vaccination helps to protect from influenza. The effect generally lasts for a year. It is recommended that an individual takes this vaccination once in a year since the strain of the virus keeps changing every year. Flu vaccine com...more
The flu vaccination helps to protect from influenza. The effect generally lasts for a year. It is recommended that an individual takes this vaccination once in a year since the strain of the virus keeps changing every year. Flu vaccine com...more
Last Updated: 7 years ago• Featured Tip
Share
Bookmark
Report
During pregnancy, flu (influenza) can impose serious health implications for both the mother and the child. Due to pregnancy, the risk of developing complications like pneumonia are very high, which can pose as a problem during childbirth. Miscarriage, low birth weight, premature birth are some of the major issues, which might develop if the mother has suffered from flu during her pregnancy. Although flu vaccination during pregnancy has certain risks, it has been observed that in most cases the ...more
Last Updated: 9 years ago• Featured Tip
Share
Bookmark
Report
During pregnancy, flu (influenza) can impose serious health implications for both the mother and the child. Due to pregnancy, the risk of developing complications like pneumonia are very high, which can pose as a problem during childbirth. Miscarriage, low birth weight, premature birth are some of the major issues, which might develop if the mother has suffered from flu during her pregnancy. Although flu vaccination during pregnancy has certain risks, it has been observed that in most cases the ...more
Last Updated: 7 years ago• Featured Tip
Share
Bookmark
Report
A common disease that can spread easily is Influenza. Commonly known as flu, the infection of influenza can adversely affect people in various ways.
However, with an annual shot of influenza vaccine, you can stay immune to the infection, and the more the number of people getting vaccinated, the lesser the number of contagious infections.
How does the influenza vaccine work?
Influenza vaccine leads to the production of antibodies in the body within two weeks after taking the...more
However, with an annual shot of influenza vaccine, you can stay immune to the infection, and the more the number of people getting vaccinated, the lesser the number of contagious infections.
How does the influenza vaccine work?
Influenza vaccine leads to the production of antibodies in the body within two weeks after taking the...more
Last Updated: 10 years ago• Featured Tip
Share
Bookmark
Report
Ask a free question
Get FREE multiple opinions from Doctors
posted anonymously